Highlighting Animals in Research
Tuberculosis Treatment [1]
 The first new drugs in 40 years to fight tuberculosis have been approved by national regulatory agencies. On December 28, 2012, the Food and Drug Administration approved bedaquiline as part of a drug regimen to treat multi-drug resistant tuberculosis. A second new drug called delamanid was approved by the European Medicines Agency on April 28, 2014. In 2015,
The first new drugs in 40 years to fight tuberculosis have been approved by national regulatory agencies. On December 28, 2012, the Food and Drug Administration approved bedaquiline as part of a drug regimen to treat multi-drug resistant tuberculosis. A second new drug called delamanid was approved by the European Medicines Agency on April 28, 2014. In 2015,  both medications were included in the Essential Medicines List of the World Health Organization. Bedaquiline and delamanid were tested in mouse and guinea pig models before moving into human clinical trials.
both medications were included in the Essential Medicines List of the World Health Organization. Bedaquiline and delamanid were tested in mouse and guinea pig models before moving into human clinical trials.
Multiple Sclerosis Treatment [2]
 On February 17, 2016, the Food and Drug Administration granted the Breakthrough Therapy Designation for ocrelizumab, a new drug that shows promise for the treatment of multiple sclerosis. The designation was for use of the drug in primary progression multiple sclerosis, although it also shows promise for other forms of the disease like relapsing multiple sclerosis. There are currently no FDA-approved treatments for primary progression multiple sclerosis, which represents about 10% of patients with the disease. Ocrelizumab (trade name Ocrevus) was tested in mice and monkeys for safety and efficacy before beginning human clinical trials.
On February 17, 2016, the Food and Drug Administration granted the Breakthrough Therapy Designation for ocrelizumab, a new drug that shows promise for the treatment of multiple sclerosis. The designation was for use of the drug in primary progression multiple sclerosis, although it also shows promise for other forms of the disease like relapsing multiple sclerosis. There are currently no FDA-approved treatments for primary progression multiple sclerosis, which represents about 10% of patients with the disease. Ocrelizumab (trade name Ocrevus) was tested in mice and monkeys for safety and efficacy before beginning human clinical trials.
Ebola Vaccine [3]
On July 25, 2016, the Food and Drug Administration and the European Medicines Agency granted Breakthrough Therapy Designation to the VSV ZEBOV vaccine to treat Ebola. The vaccine was  developed in mice and proven to be safe and effective in monkeys, paving the way for preliminary human clinical trials and fast-track development. Ebola is a deadly hemorrhagic virus that broke out most recently in West Africa in 2014, directly causing over 10,000 reported deaths. The vaccine could help prevent future outbreaks and provides a new model for vaccine development to address highly infectious diseases.
developed in mice and proven to be safe and effective in monkeys, paving the way for preliminary human clinical trials and fast-track development. Ebola is a deadly hemorrhagic virus that broke out most recently in West Africa in 2014, directly causing over 10,000 reported deaths. The vaccine could help prevent future outbreaks and provides a new model for vaccine development to address highly infectious diseases.
Cystic Fibrosis Treatment [4]
 On July 2, 2015, the FDA approved the combination drug lumacaftor/ivacaftor (trade name Orkambi), the first drug to address the genetic mutations that cause cystic fibrosis (CF), for children and adults ages 12 and up. On September 27, 2016, the drug was approved for younger patients above the age of six as well. More than one thousand genetic mutations have been identified in the development of CF. The new drug addresses the most common – two copies of the F508del mutation in the CFTR gene that accounts for about half of CF cases – and holds promise for thousands of people affected by the disease. Orkambi was tested in mice and rats before beginning human clinical trials.
On July 2, 2015, the FDA approved the combination drug lumacaftor/ivacaftor (trade name Orkambi), the first drug to address the genetic mutations that cause cystic fibrosis (CF), for children and adults ages 12 and up. On September 27, 2016, the drug was approved for younger patients above the age of six as well. More than one thousand genetic mutations have been identified in the development of CF. The new drug addresses the most common – two copies of the F508del mutation in the CFTR gene that accounts for about half of CF cases – and holds promise for thousands of people affected by the disease. Orkambi was tested in mice and rats before beginning human clinical trials.
Wireless Pacemaker [5]
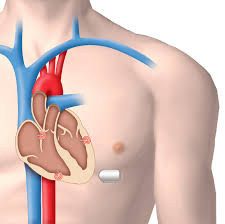 Electronic pacemakers to regulate an irregular heartbeat have been around for over fifty years. Until now, the surgical procedure to implant the device has remained relatively similar: a small incision is made in the chest, and the device is guided into position using small wires. It is these wires that have caused the most complications related to the device, sometimes tearing nearby tissues or causing massive infections. But those wired pacemakers are now a thing of the past. On April 6, 2016, the Food and Drug Administration approved the first wireless pacemaker, which uses a catheter inserted in the groin to guide the device into place without wires. Human clinical trials showed that the new device was just as effective at correctly pacing the heart as the current wired pacemakers, but the wireless pacemaker has the potential to greatly reduce complications. The new wireless pacemaker was tested in dogs, sheep and pigs to ensure safety and efficacy before human trials began.
Electronic pacemakers to regulate an irregular heartbeat have been around for over fifty years. Until now, the surgical procedure to implant the device has remained relatively similar: a small incision is made in the chest, and the device is guided into position using small wires. It is these wires that have caused the most complications related to the device, sometimes tearing nearby tissues or causing massive infections. But those wired pacemakers are now a thing of the past. On April 6, 2016, the Food and Drug Administration approved the first wireless pacemaker, which uses a catheter inserted in the groin to guide the device into place without wires. Human clinical trials showed that the new device was just as effective at correctly pacing the heart as the current wired pacemakers, but the wireless pacemaker has the potential to greatly reduce complications. The new wireless pacemaker was tested in dogs, sheep and pigs to ensure safety and efficacy before human trials began.
New Class of Cholesterol-Reducing Drugs [6]
 The FDA has approved a new class of drugs shown to be more effective at lowering cholesterol than statins. The FDA approved alirocumab (trade name Praluen) on July 24, 2015, and evolocumab (trade name Repatha) on August 27, 2015. They are both
The FDA has approved a new class of drugs shown to be more effective at lowering cholesterol than statins. The FDA approved alirocumab (trade name Praluen) on July 24, 2015, and evolocumab (trade name Repatha) on August 27, 2015. They are both  Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors. The drugs were tested for safety and efficacy in rats and monkeys before moving into human clinical trials.
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors. The drugs were tested for safety and efficacy in rats and monkeys before moving into human clinical trials.
Bionic Eye [7]
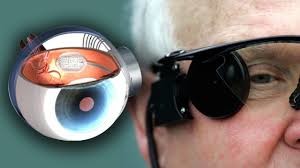 This new device to restore sight is as futuristic as it sounds. Specialized glasses worn by the patient transmit visual information to a small device implanted behind the eye. This device then stimulates the nearby cells to transmit the visual information to the brain. The Food and Drug Administration approved the Argus II Retinal Prosthesis System on February 14, 2013, which was successfully implanted in patients one year later. The Bionic Eye was first tested and optimized in mice and rats before moving into human clinical trials.
This new device to restore sight is as futuristic as it sounds. Specialized glasses worn by the patient transmit visual information to a small device implanted behind the eye. This device then stimulates the nearby cells to transmit the visual information to the brain. The Food and Drug Administration approved the Argus II Retinal Prosthesis System on February 14, 2013, which was successfully implanted in patients one year later. The Bionic Eye was first tested and optimized in mice and rats before moving into human clinical trials.
New Treatment for Drug-Resistant Epilepsy [8]
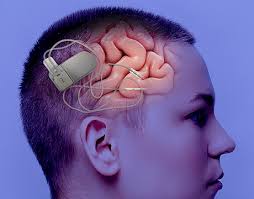 About 400,000 adults in the United States suffer from regular epileptic seizures that current medications cannot successfully control. Approved by the Food and Drug Administration on November 14, 2013, the NeuroPace System offers a new option for epilepsy patients. The electronic device and several wires are implanted into the brain, gathering brain-wave data. When the device detects an abnormal pattern, it stimulates the affected part of the brain to prevent seizures. For patients who have already tried anti-epileptic drugs without relief, this is an exciting new option that could improve daily life. The NeuroPace System was tested in guinea pigs and sheep for safety and efficacy before beginning human clinical trials.
About 400,000 adults in the United States suffer from regular epileptic seizures that current medications cannot successfully control. Approved by the Food and Drug Administration on November 14, 2013, the NeuroPace System offers a new option for epilepsy patients. The electronic device and several wires are implanted into the brain, gathering brain-wave data. When the device detects an abnormal pattern, it stimulates the affected part of the brain to prevent seizures. For patients who have already tried anti-epileptic drugs without relief, this is an exciting new option that could improve daily life. The NeuroPace System was tested in guinea pigs and sheep for safety and efficacy before beginning human clinical trials.
Preventing HIV Transmission [9]
 On July 16, 2012, the Food and Drug Administration approved the first drug to prevent the sexual transmission of HIV infection. The drug, a combination treatment called emtricitabine/tenofovir (trade name Truvada) has been approved for pre-exposure prophylaxis (PReP) in people who are at high risk of contracting HIV. About 50,000 adults are newly diagnosed with HIV each year, and there is currently no cure for the disease. The development of effective methods to prevent transmission is an important step in the fight against HIV. The drug was tested in mice, rats and rabbits before beginning human clinical trials.
On July 16, 2012, the Food and Drug Administration approved the first drug to prevent the sexual transmission of HIV infection. The drug, a combination treatment called emtricitabine/tenofovir (trade name Truvada) has been approved for pre-exposure prophylaxis (PReP) in people who are at high risk of contracting HIV. About 50,000 adults are newly diagnosed with HIV each year, and there is currently no cure for the disease. The development of effective methods to prevent transmission is an important step in the fight against HIV. The drug was tested in mice, rats and rabbits before beginning human clinical trials.
Notes
[1] http://www.npr.org/sections/health-shots/2014/12/03/367814133/in-new-york-video-chat-trumps-quarantine-to-combat-tb. Credit: Science Photo Library/Corbis (lungs); https://www.britannica.com/animal/guinea-pig. Credit: Erik Lam/Shuttershock.com (guinea pigs).
[2] https://www.benaroyaresearch.org/what-is-bri/disease-information/multiple-sclerosis#.WHlBbn2zmuw
[3] https://www.jax.org/news-and-insights/2011/january/diabetes-mouse-models-used-as-models-for-wound-healing
[4] https://bionews-tx.com/news/2014/03/04/scientists-identify-105-new-inheritable-genetic-mutations-causing-cystic-fibrosis/
[5] http://www.implantable-device.com/2011/12/15/mtcs-piezos-used-for-wireless-power-transmission-in-ebrs-wireless-pacemaker/ (device); http://www.ldoceonline.com/dictionary/sheep (sheep)
[6] http://www.ozonetherapymalaysia.com/how-to-lower-cholesterol/; http://www.understandinganimalresearch.org.uk/animals/types-animals/research-using-monkeys/ (monkey)
[7] https://i.ytimg.com/vi/Bh3MaoPVdNM/maxresdefault.jpg
[8] https://www.asme.org/engineering-topics/articles/bioengineering/treating-body-mind-epilepsy-patients
